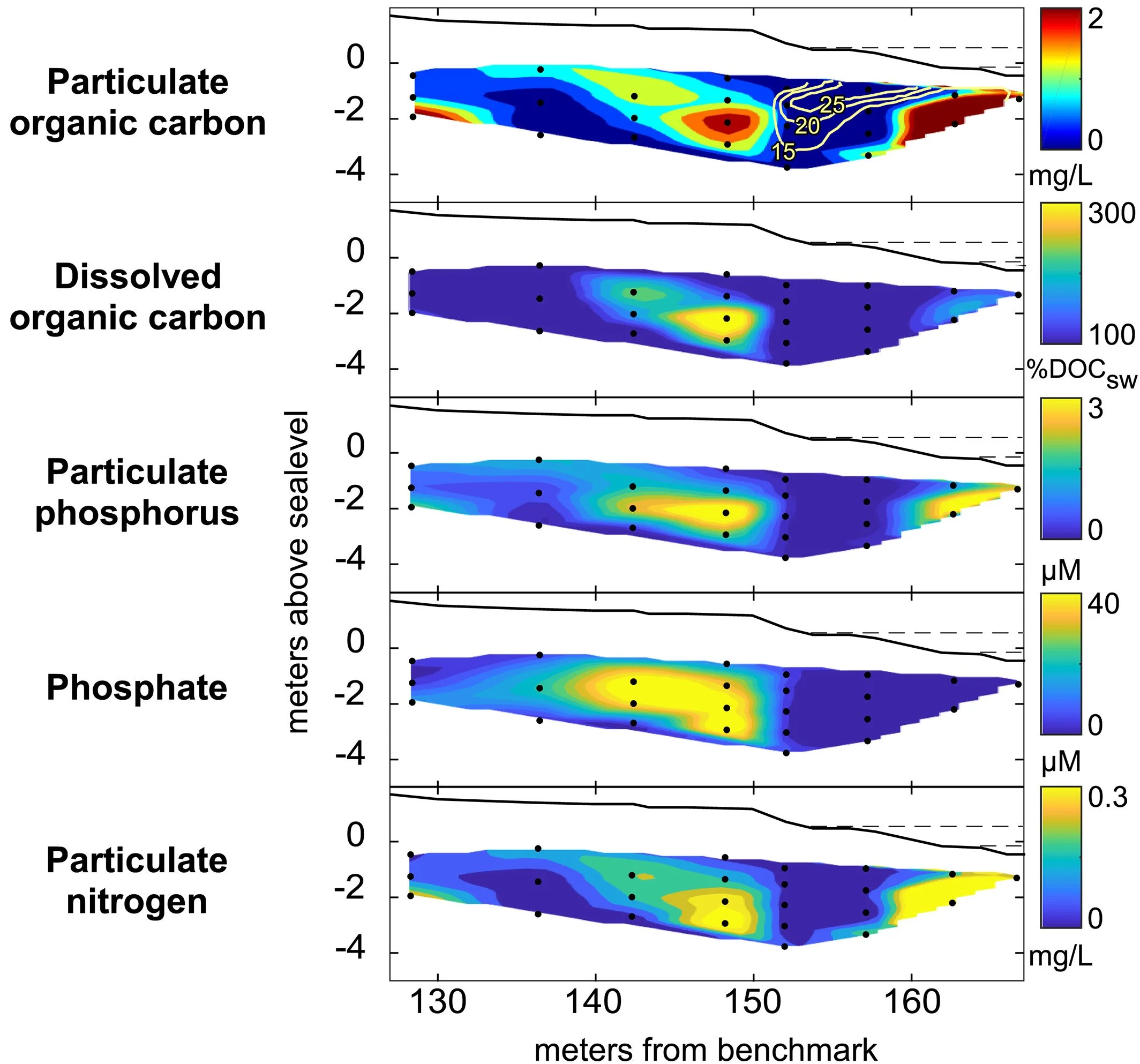
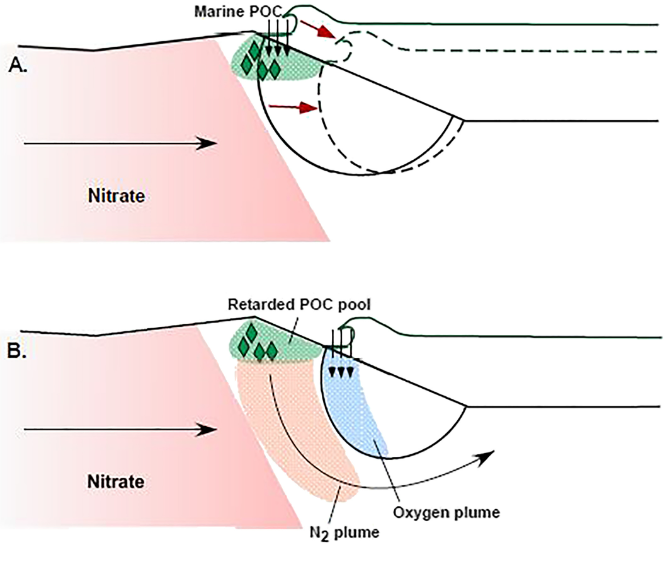
Results support a “carbon memory” effect within the beach, with the evolution and migration of reaction patterns relating to the distribution of these scattered carbon pools as more mobile solutes move over them during changes in hydrologic conditions.